Recombinant Conigen Soluble Proteins Mimicking Native Conformations
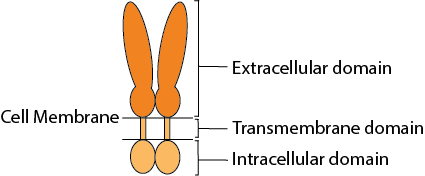
The CSP technology platform consists of soluble recombinant dimeric and trimeric proteins with a quaternary structure mimicking the natural conformation of protein complexes on cell surfaces, such as receptors or cancer target proteins. These recombinant dimers and trimers enable more biologically relevant translational research and therapeutic antibody discovery.
Ready-to-go advanced multimeric soluble proteins: antigen and immunogen applications
| Protein | Protein Type | Description | Product Code | Quantities | Details |
|---|---|---|---|---|---|
| Human CD4 dimer | Recombinant homodimer protein | CD4 extracellular 4 domains (Lys26-Phe396) with a homodimer motif and His tag at C-terminus | CSP-24004 | 20µg, 50µg, 100µg, 250µg, 1mg | View Details |
| Human IFNγR1 Dimer | Recombinant homodimer protein | IFNγR1, 2 extracellular domains (amino acids Glu18-Gly245) with a homodimer motif and a His tag at the C-terminus | CSP-24015 | 20µg, 50µg, 100µg, 250µg, 1mg | View Details |
| Human IL10Rα | Recombinant homodimer protein | IL10Rα, 2 IL10Rα extracellular domains (amino acids His22-Asn235) with a homodimer motif and a His tag at the C-terminus | CSP-24018 | 20µg, 50µg, 100µg, 250µg, 1mg | View Details |
| Human TIGIT Dimer | Recombinant homodimer protein | TIGIT extracellular domain (amino acids Met22-Phe141) with a homodimer Fc tag at the C-terminus | CSP-24028 | 20µg, 50µg, 100µg, 250µg, 1mg | View Details |
Combining multiple proprietary advanced protein engineering approaches to:
- Conformation: Novel multimerization interfacing element creating native ligand binding site
- Stability: Increased stability of multimeric form via stabilization element
- Productivity: Increased protein secretion during expression in mammalian cells
Why are soluble multimeric proteins needed ?
Key design elements for recombinant conformational multimeric proteins
- Recombinant molecule design – target sequence, multimerization interface and stabilization elements
- Construct design – increased protein secretion
- Present better conformational epitopes compared to currently available monomeric molecules
- Enhanced bioactivity
Flexible CSP Technology Platform is capable of creating a variety of modes of functional multimeric protein receptors
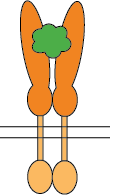
Ligand interacts with homo-dimer protein receptor
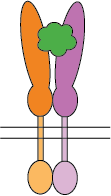
Ligand interacts with hetero-dimer protein receptor
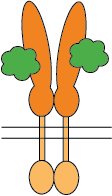
Two ligands interacting with homo-dimer protein chains
Ligand interacts with homo-trimer protein chains
The self-association of proteins to form dimers, trimers, and higher-order oligomers on cell surfaces as integral membrane proteins is a very common configuration for receptors and bioactive molecules. The extracellular domains are mostly responsible for ligand/receptor interactions and antibody binding which impacts downstream functional activities. To evaluate the bioactivities of such molecules, cell-based assays or recombinant proteins of extracellular domains are often used. However, one of the pitfalls of cell-based assays is that there isn’t always a readily available corresponding cell line for every target. The extracellular domain recombinant proteins on the market are limited to monomeric proteins which eliminate critical conformational epitopes on the quaternary structure of the monomeric or higher-order oligomeric molecules. The CSP Technology Platform provides native conformational epitopes with enhanced bioactivity, compared to currently available monomeric molecules, enabling more biologically relevant translational research and therapeutic antibody discovery.
Class of proteins: cytokine receptors, immune-oncology targets, immune checkpoints, T and B cell modulatory molecules
Example: CD4 dimer
CSP Applications
Conigen specializes in engineering dimeric or trimeric soluble recombinant proteins to mimic the natural conformation of the dimeric and trimeric molecules on cell surfaces. These multimeric soluble recombinant proteins can be used for applications including in vitro bioactivity assays and in vivo for animal (RUO) immunizations.
CSP Advantages
Comparing to cell-based assays for bioactivity
- Cell line isn’t always available for the target of interest
- CSP can preserve the native conformations similar to those on cell surfaces
- Easy to scale-up and high-through-put assays
Comparing to monomeric proteins of the extracellular domains for bioactivity measurements
- CSP not limited to monomeric proteins without critical conformational epitopes on the quaternary structures
- Provides better conformational epitopes for antibody screening and affinity evaluation
- Enhances assay sensitivity and potency; generates wider windows for agonist and antagonist activity evaluation
- Better conformation for antibody/antigen, receptor/ligand complex structural analysis
Comparing to whole cell immunization
- Preserves the native conformations similar to those on cell surfaces
Example Multimeric Soluble Protein: CD4 dimer
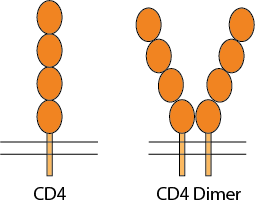
CD4 is a 55-kDa type I membrane-associated glycoprotein, expressed at the surface of thymocytes, helper T lymphocytes, macrophages/monocytes, and hematopoietic progenitor cells, as a cell marker and receptor for IL16 cytokine and HIV viruses. CD4 molecules on the cell surface can be in a monomer form, dimer form, or in a complex with T cell receptors. CD4 for functional activities as a receptor has the tendency to be in a dimeric form. The CD4 molecule has 4 extracellular Ig-like domains, a transmembrane domain and an intracellular domain. The extracellular domains are responsible for ligand binding, such as HIV-1 envelop protein gp120 which then mediates the HIV-1 infection, and IL16 binding to trigger immune signaling pathways. The homodimer or oligomerization state of CD4 receptors on the cell surface is believed to play an important role in pathophysiological processes, such as binding to MHC-II, T-cell activation and HIV infection.
To better mimic the conformation of dimeric human CD4 on the cell surface, Conigen has engineered a novel CD4 soluble dimeric form of the protein with 4 extracellular domains (amino acids Lys26-Phe396; Accession# A0A4Y5UGE4), a proprietary dimeric motif at the C-terminus of D4, and a C-terminus His-Tag for affinity purification.
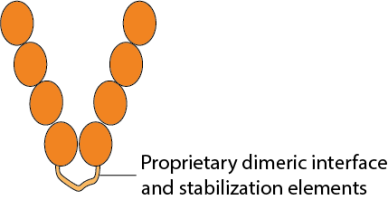
This CD4 soluble dimer molecule from Conigen has a proprietary dimeric interface and stabilization elements at the C-terminus of the CD4 extracellular domain (ECD) that is engineered by genetic modification of the DNA coding sequence which is then translated as one polypeptide chain. During expression in mammalian cells, two identical polypeptide chains are associated by the dimer motif to form a soluble CD4-ECD homodimer. The dimeric motif design is intended to form a homodimer, preserving the native conformation and thereby preventing potential structural disorder caused by tangling of the two chains.
Engineered soluble CD4 dimer protein
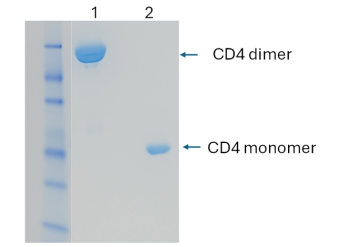
The engineered CD4 dimer protein is expressed in HEK293 cells and purified as a dimeric form (lane 1), demonstrated by SDS-PAGE.
CD4 dimer binding to HIV-1 gp120 is >10-fold more potent than CD4 monomer
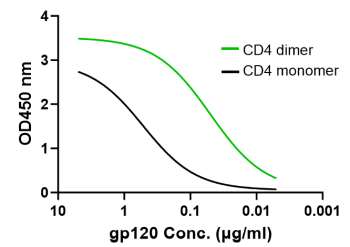
Dimeric CD4 (green, EC50 0.05 µg/ml) significantly enhances the binding to HIV-1 subtype B isolate AC10.29 gp120 protein versus the monomeric CD4 (black) form (EC50 0.52 µg/ml). Similar patterns were demonstrated binding gp120 proteins from different HIV-1 subtypes (i.e., B, BC and AE), up to 40-fold EC50 difference between CD4 dimer and monomer.
CD4 dimer preserves the desirable conformations for gp120 binding
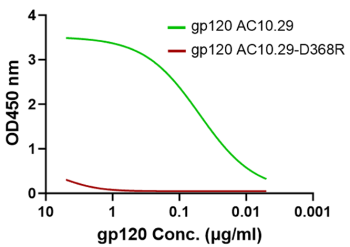
CD4 binding site mutation D368R (red) diminishes the gp120 binding to the CD4 protein dimer which proves that the CD4 dimer (green) has the proper conformation as the receptor for the HIV-1 envelope glycoprotein.
CD4 dimer significantly increases the binding to CD4-specific antibodies
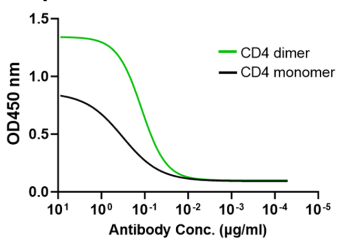
CD4 dimer (green) significantly increases the binding to CD4-specific human monoclonal antibody (Keliximab) compared to CD4 monomer.
FAQs
How stable are the multimeric soluble proteins?
The stability of the purified recombinant multimeric proteins is comparable to the stability of other recombinant proteins. These proteins are stable for at least 6 months when frozen (-70°C) or at least 7 days at 4°C. Like most recombinant proteins, excessive vortexing, dilution, and freeze-thaw should be avoided to minimize sample and activity loss. More information can be found on the datasheet.
Can multimeric soluble proteins be labeled or modified?
The recombinant multimeric proteins can readily be modified. Please contact Conigen to request tag-free, a special tag, biotinylated or fluorescently labeled molecules.
What quality control standards are used for the multimeric soluble proteins?
The Conigen validation process involves various analytical techniques for each batch, including techniques such as SDS-PAGE, Western blot, and ELISA
How do I order multimeric soluble proteins?
Please contact Conigen using the Consultation request form.
How do I order custom services for multimeric soluble proteins?
Please contact Conigen using the Consultation request form.
What is the lead time for custom services for soluble proteins?
The turnaround time of custom orders is approximately 6-8 weeks from sequence to product. Please contact Conigen using the Consultation request form for more information.
References
- Wright GJ. 2009. Signal initiation in biological systems: the properties and detection of transient extracellular protein interactions. Mol Biosyst 5:1405-12.
- Brown JH. 2006. Breaking symmetry in protein dimers: designs and functions. Protein Sci 15:1-13.
- Atanasova M, Whitty A. 2012. Understanding cytokine and growth factor receptor activation mechanisms. Crit Rev Biochem Mol Biol 47:502-30.
- Mei G, Di Venere A, Rosato N, Finazzi-Agro A. 2005. The importance of being dimeric. FEBS J 272:16-27.
- Marianayagam NJ, Sunde M, Matthews JM. 2004. The power of two: protein dimerization in biology. Trends Biochem Sci 29:618-25.
- Maekawa A, Schmidt B, Fazekas de St Groth B, Sanejouand YH, Hogg PJ. 2006. Evidence for a domain-swapped CD4 dimer as the coreceptor for binding to class II MHC. J Immunol 176:6873-8.
- Matthias LJ, Azimi I, Tabrett CA, Hogg PJ. 2010. Reduced monomeric CD4 is the preferred receptor for HIV. J Biol Chem 285:40793-9.
- Moldovan MC, Sabbagh L, Breton G, Sekaly RP, Krummel MF. 2006. Triggering of T cell activation via CD4 dimers. J Immunol 176:5438-45.
- Li S, Satoh T, Korngold R, Huang Z. 1998. CD4 dimerization and oligomerization: implications for T-cell function and structure-based drug design. Immunol Today 19:455-62.
- Langedijk JP, Puijk WC, van Hoorn WP, Meloen RH. 1993. Location of CD4 dimerization site explains critical role of CDR3-like region in HIV-1 infection and T-cell activation and implies a model for complex of coreceptor-MHC. J Biol Chem 268:16875-8.
- Center DM, Kornfeld H, Ryan TC, Cruikshank WW. 2000. Interleukin 16: implications for CD4 functions and HIV-1 progression. Immunol Today 21:273-80.
