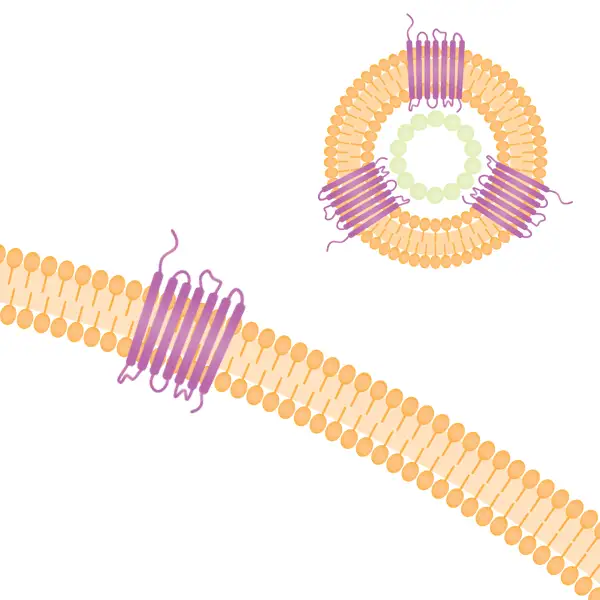
Full length multi-pass target proteins embedded in lipid bilayer of virus-like nanoparticles (VLPs) Conigen Membrane Protein Nanoparticles
The CMP™ platform enables expression of full-length, multi-pass membrane proteins with preserved structure and function. Using a virus-like particle (VLP) format, these nanoparticles display higher density target membrane proteins with a native conformation and are expressed in mammalian cells to preserve the natural post-translational process. The viral capsid backbone is engineered to increase VLP release for higher production yield.
These proteins are ideal for functional studies, antibody discovery, and drug screening.
How it Works
Combining multiple proprietary protein engineering techniques for:
- Higher density: Enhance cell-surface expression to produce nanoparticles with higher density multipass membrane protein molecules
- Better immunogenicity: Incorporate immune-engaging elements for immunogen applications
- Functionality: Maintain membrane protein bioactivities via design considerations
Why are new antigen and immunogen formats for complex membrane proteins needed?
Membrane proteins embedded within lipid bilayer-based cell plasma membranes make up large portions of human proteome
Receptors, transporters, ion channels and enzymes related to cell membranes mediate interactions between cells and their environment
More than 60% of the current drugs target membrane proteins
Challenges of working with complex membrane proteins
Membrane proteins are not typically abundant on the cell surface making them very difficult to purify and characterize
Membrane proteins are structurally complex, taking on various conformational forms
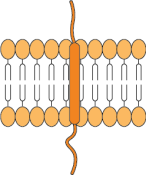
Single transmembrane domain
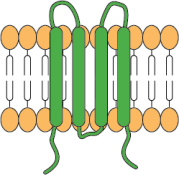
4-transmembrane domains
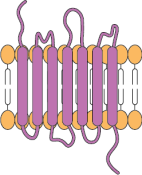
7-transmembrane domains
Categories of complex membrane proteins can be expressed using the CMP technology platform: GPCRs, Claudin family, ion channels, and immuno-oncology targets
Scientific Rationale
Membrane proteins are central to drug targeting and immune recognition, yet difficult to express in functional form. Many methods, including detergent solubilization or peptide synthesis distort structural integrity and epitope presentation.
The CMP™ technology platform resolves these challenges by preserving native folding and membrane context. This supports:
- Better antigenicity: Immunogens to generate better antibodies targeting conformational epitopes
- Reliable antigen format: Antigens to screen and characterize antibodies
- Increased sensitivity: Drug screening and bioactivity evaluation with greater sensitivity and broader dynamic range
- Agonist: confirms drug binding ability to receptor
- Antagonist: measures ability of drug to block receptor/ligand interactions
- Robust tools: For structural analyses
CMP Advantages
CMP vs reconstituted membrane-lipid-belt protein formats (Nanodiscs, liposomes)
- More natively folded proteins in the cellular membrane bilayer without further processing
CMP vs peptides immunization
- Eliciting antibodies that better target conformational epitopes
CMP vs whole cell immunization
- Higher antigen dosage per immunization
- Reduced number of host cell derived proteins
- More immunogenic
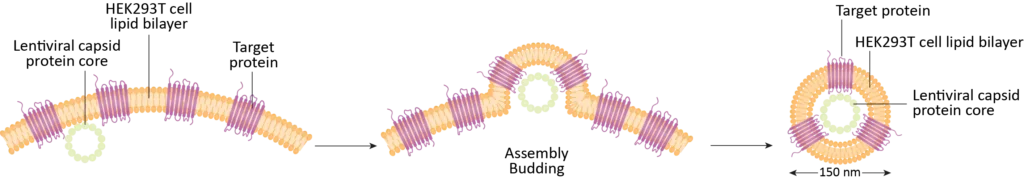
Example Membrane Protein Nanoparticle: CXCR4 (7-transmembrane domain GPCR)
The cell surface C-X-C chemokine receptor 4 (CXCR4) is a seven transmembrane domain G protein-coupled receptor (GPCR), expressed on white blood cells and hematopoetic stem cells. CXCL12 is the endogenous chemokine ligand of CXCR4, and the CXCL12/CXCR4 complex activates various signaling pathways promoting chemotaxis, adhesion and migration, cell proliferation and survival. This receptor also acts with the CD4 protein as a co-receptor to mediate HIV entry into cells.
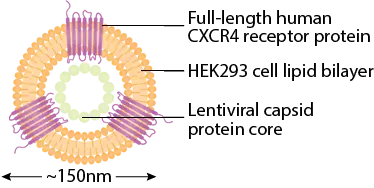
- Human full-length protein anchored in the lipid bilayer produced by HEK293 cells
- Lentiviral capsid protein core without viral genetic materials
- Spherical nanoparticles with diameter ~150nm
- Bioactive CXCR4 molecules bind to ligand CXCL12 and anti-CXCR4 antibodies
Performance Data
CXCR4 Specific Antibody Binding
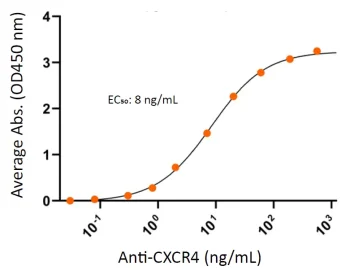
Human CXCR4 protein CMP (CMP-24005) potently binds to CXCR4 specific monoclonal antibody (mAb) as measured by ELISA. The CXCR4 protein dimer (0.5 μg/ml) was coated on 96-well microtiter plates and detected by serial dilutions of anti-human CXCR4 mAb.
CXCR4 Binding to CXCL12 Chemokine
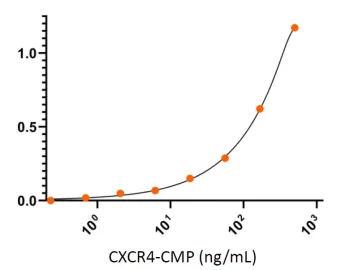
Human CXCR4 protein CMP (CMP-24005) binds well to its ligand CXCL12 chemokine as measured by ELISA. The CXCL12 protein dimer (0.5 μg/ml) was coated on 96-well microtiter plates and detected by serial dilutions of CXCR4-CMP.
CXCR4 Binding to HIV-1 gp120
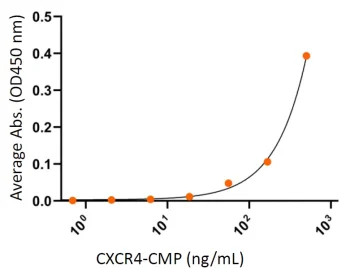
Human CXCR4 protein CMP (CMP-24005) binds well to its ligand HIV-1 envelope glycoprotein gp120 as measured by ELISA. The gp120 protein dimer (0.5 μg/ml) was coated on 96-well microtiter plates and detected by serial dilutions of CXCR4-CMP.
FAQs
How stable are the nanoparticles?
The nanoparticle stability is comparable to the stability of other purified proteins. Nanoparticles are stable for at least 6 month when frozen (-70°C) or at least 14 days at 4°C. Like most lipid structures, nanoparticles will be disrupted by detergents. Nanoparticle suspensions will appear as an opaque solution and should be gently mixed before use. Excessive vortexing, dilution, and filtration of nanoparticles should be avoided to minimize sample loss. More information can be found on the datasheet.
Can nanoparticles be labeled or modified?
The nanoparticles can readily be modified during the production and purification process. Please contact Conigen to request biotinylated or fluorescently labeled molecules.
Do nanoparticles contain nonspecific membrane proteins?
As nanoparticles bud from the cell surface, they contain high-copy numbers of specific target membrane protein of interest in addition to membrane proteins from the cells that are used for production. The effects of these nonspecific membrane proteins are minimized by the overexpression of the target membrane protein of interest. In your experimental set-up, Conigen recommends the following negative controls: alternative cell types, and the use of null nanoparticles that do not contain the target membrane protein of interest.
What is the size of the nanoparticles?
Generally, nanoparticles range in size (diameter) from approximately 100 to 300nm.
What quality control tests are used for the nanoparticle-displayed antigens?
The Conigen validation process involves various analytical techniques for each batch, including techniques such as dynamic light scatter, Western blot, and ELISA
How do I order membrane proteins?
Please contact Conigen at [email protected] to place an order via PO.
How do I order custom services for membrane proteins?
Please contact Conigen using the Consultation request form.
What is the lead time for custom services for membrane proteins?
The turnaround time of custom orders is approximately 6-8 weeks from sequence to product. Please contact Conigen using the Consultation request form for more information.
References
- Smalinskaite L, Kim MK, Lewis AJO, Keenan RJ, Hegde RS. 2022. Mechanism of an intramembrane chaperone for multipass membrane proteins. Nature 611:161-166.
- Hegde RS, Keenan RJ. 2022. The mechanisms of integral membrane protein biogenesis. Nat Rev Mol Cell Biol 23:107-124.
- Gardill B, Huang J, Tu L, Van Petegem F, Oxenoid K, Thomson CA. 2020. Nanodisc technology facilitates identification of monoclonal antibodies targeting multi-pass membrane proteins. Sci Rep 10:1130.
- Jo M, Jung ST. 2016. Engineering therapeutic antibodies targeting G-protein-coupled receptors. Exp Mol Med 48:e207.
- Mohsen MO, Bachmann MF. 2022. Virus-like particle vaccinology, from bench to bedside. Cell Mol Immunol 19:993-1011.
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. 1998. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393:595-9.
- Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC. 2010. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330:1066-71.
- Shaik MM, Peng H, Lu J, Rits-Volloch S, Xu C, Liao M, Chen B. 2019. Structural basis of coreceptor recognition by HIV-1 envelope spike. Nature 565:318-323.
- Wald O, Izhar U, Amir G, Kirshberg S, Shlomai Z, Zamir G, Peled A, Shapira OM. 2011. Interaction between neoplastic cells and cancer-associated fibroblasts through the CXCL12/CXCR4 axis: role in non-small cell lung cancer tumor proliferation. J Thorac Cardiovasc Surg 141:1503-12.
- Guo F, Wang Y, Liu J, Mok SC, Xue F, Zhang W. 2016. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene 35:816-26.
- Huang MB, Giesler KE, Katzman BM, Prosser AR, Truax V, Liotta DC, Wilson LJ, Bond VC. 2018. Small molecule CXCR4 antagonists block the HIV-1 Nef/CXCR4 axis and selectively initiate the apoptotic program in breast cancer cells. Oncotarget 9:16996-17013.
Learn more about Membrane Proteins

Poster: Recombinant G-Protein Coupled Chemokine Receptors CXCR4 and CXCR5 on Nanoparticles are Bioactive
Recombinant G-Protein Coupled Chemokine Receptors CXCR4 and CXCR5 on Nanoparticles are Bioactive, Binding to the Chemokine Ligands Download the Poster Poster Abstract The selective chemokine

CXCR5: A Critical Regulator in Immune Organization, Autoimmunity, and Cancer Therapy
CXCR5, or C-X-C Chemokine Receptor Type 5, is a G-protein-coupled receptor (GPCR) primarily known for its role in guiding B cells to lymphoid tissues and
CXCR4: A Critical Player in Cancer, Immunity, and Therapeutic Innovation
CXCR4, or C-X-C Chemokine Receptor Type 4, is a highly conserved G-protein-coupled receptor (GPCR) primarily involved in cellular migration, immune responses, and tissue repair. CXCR4

Leveraging Nature’s Blueprints for Breakthrough Bioactive Claudin Integral Membrane Proteins
Nature holds the blueprint for many of science’s most promising therapeutic solutions. At Conigen, we’ve harnessed these native designs to introduce our latest Claudin protein