Predicted Molecular Weight: 99 kDa
SDS PAGE Molecular Weight: The migration range of the dimer protein with glycosylation under non-reducing condition is ~190 kDa on SDS PAGE.
Protein Construct: FGFR1 dimer protein contains a FGFR1 extracellular domainfused with a proprietary dimer motif followed by a tandem His-Avi tag at the C-terminus.
Human fibroblast growth factor receptor 1 (FGFR1) is a cell surface receptor belonging to the immunoglobulin superfamily and a transmembrane receptor tyrosine kinase (RTK) that belongs to the FGFR family. FGFR1 is also known as basic fibroblast growth factor receptor 1 (BFGFR), cluster of differentiation 331 (CD331), CEK, FGFBR, FLG, Fms-like tyrosine kinase 2 (FLT-2), HBGFR, HH2, HRTFDS, KAL2, N-SAM, OGD, and ECCL. FGFR1, a Type I transmembrane protein, contains an extracellular domain with three immunoglobulin-like (Ig-like) subdomains (D1, D2 and D3), followed by a transmembrane, and an intracellular domain. Dimerization of FGFRs is necessary for activation and they can homodimerize and heterodimerize in both the presence and absence of ligand. FGFRs bind fibroblast growth factors (FGFs) leading to phosphorylation and triggering signaling cascades. FGFR1 overexpression has been reported in a variety of cancers including up to 22% of non-small-cell lung carcinoma, 14% of urinary bladder transitional cell carcinomas, 10% of estrogen receptor positive breast cancers, and 10% of squamous cell head and neck cancers. Ligand-independent FGFR1 dimerization is an important driver of hematologic malignancies, solid tumors, and developmental disorders. Inhibition of FGFRs has been an emerging target for cancer therapeutics, therefore, a recombinant protein mimicking the FGFR1 dimer conformation can be critical for cancer therapeutic discovery.
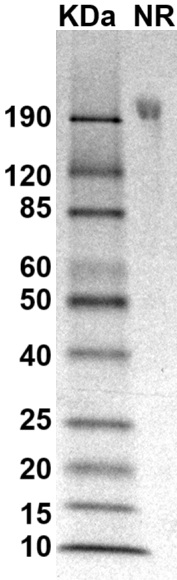 MW: Molecular Weight marker reduced condition
NR: FGFR1 dimer under non-reduced condition
MW: Molecular Weight marker reduced condition
NR: FGFR1 dimer under non-reduced condition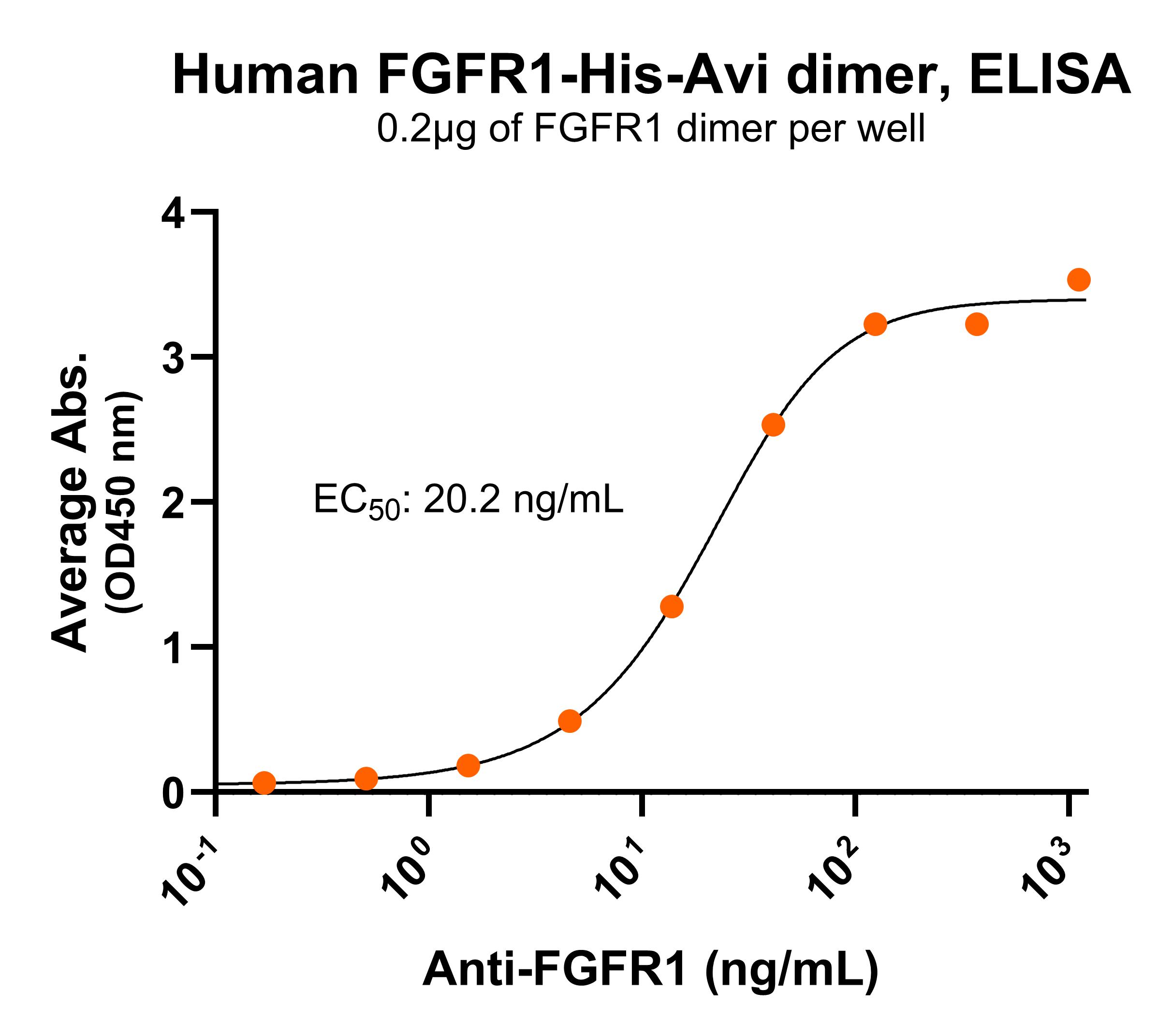 Immobilized human FGFR1-His-Avi dimer protein (CSP-25129-03) at 2 μg/mL (100 μL/well) can bind anti-human FGFR1 monoclonal antibody with half maximal effective concentration (EC50) range of 10.1-40.4 ng/mL (QC tested).
Immobilized human FGFR1-His-Avi dimer protein (CSP-25129-03) at 2 μg/mL (100 μL/well) can bind anti-human FGFR1 monoclonal antibody with half maximal effective concentration (EC50) range of 10.1-40.4 ng/mL (QC tested).