Product Name: IL6R / gp130
Predicted Molecular Weight: 120 kDa
SDS PAGE Molecular Weight: The migration range of the heterodimer protein with glycosylation under non-reducing condition is ~190 kDa; under reducing condition, the migrations of 2 chains are between 60-120 kDa and between 25-40 kDa on SDS PAGE.
Protein Construct: IL-6R heterodimer protein contains an IL-6Rα extracellular domainand gp130 extracellular domainfused with a proprietary dimer motif followed by a His tag at the IL-6Rα C-terminus and a Strep tag at the gp130 C-terminus.
Interleukin 6 receptor (IL-6R) is a heterodimer consisting of IL-6Rα and gp130 (IL-6Rβ). Both IL-6Rα and gp130 are Type 1 transmembrane proteins. IL-6Rα is Type 1 cytokine receptor and gp130 is a member of the class of tall cytokine receptors. IL-6Rα contains an extracellular domain with an Ig-like domain, cytokine binding module (CBM) domains, and a long flexible stalk region followed by a transmembrane domain and intracellular domains. The extracellular domain of gp130 includes an N-terminal immunoglobulin-like (Ig-like) domain (D1), a cytokine-binding homology region (CHR, D2D3), and three membrane-proximal fibronectin type III domains (FNIII, D4 to D6). IL-6Rα binding to its ligand interleukin 6 (IL-6) results in homodimerization and subsequent association with gp130 homodimer resulting in higher order complexes. The interaction between IL-6 cytokine and IL-6R is crucial for immune responses, inflammation, and hematopoiesis. Dysregulation of IL-6R is implicated in many cancers and autoimmune diseases. While structurally and functionally similar to human IL-6R heterodimer, mouse IL-6R heterodimer is a species-specific tool essential for preclinical studies, basic research, and translational research.
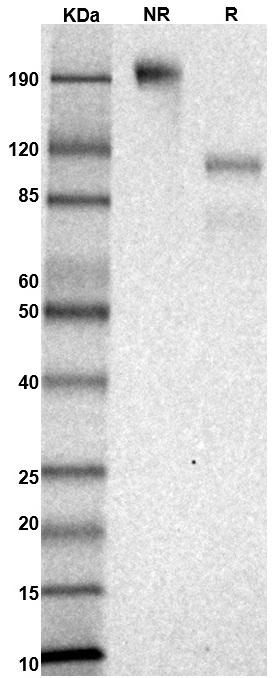 MW: Molecular Weight marker reduced condition
NR: IL-6Rα/gp130 heterodimer under non-reduced condition
R: IL-6Rα/gp130 heterodimer under reduced condition
MW: Molecular Weight marker reduced condition
NR: IL-6Rα/gp130 heterodimer under non-reduced condition
R: IL-6Rα/gp130 heterodimer under reduced condition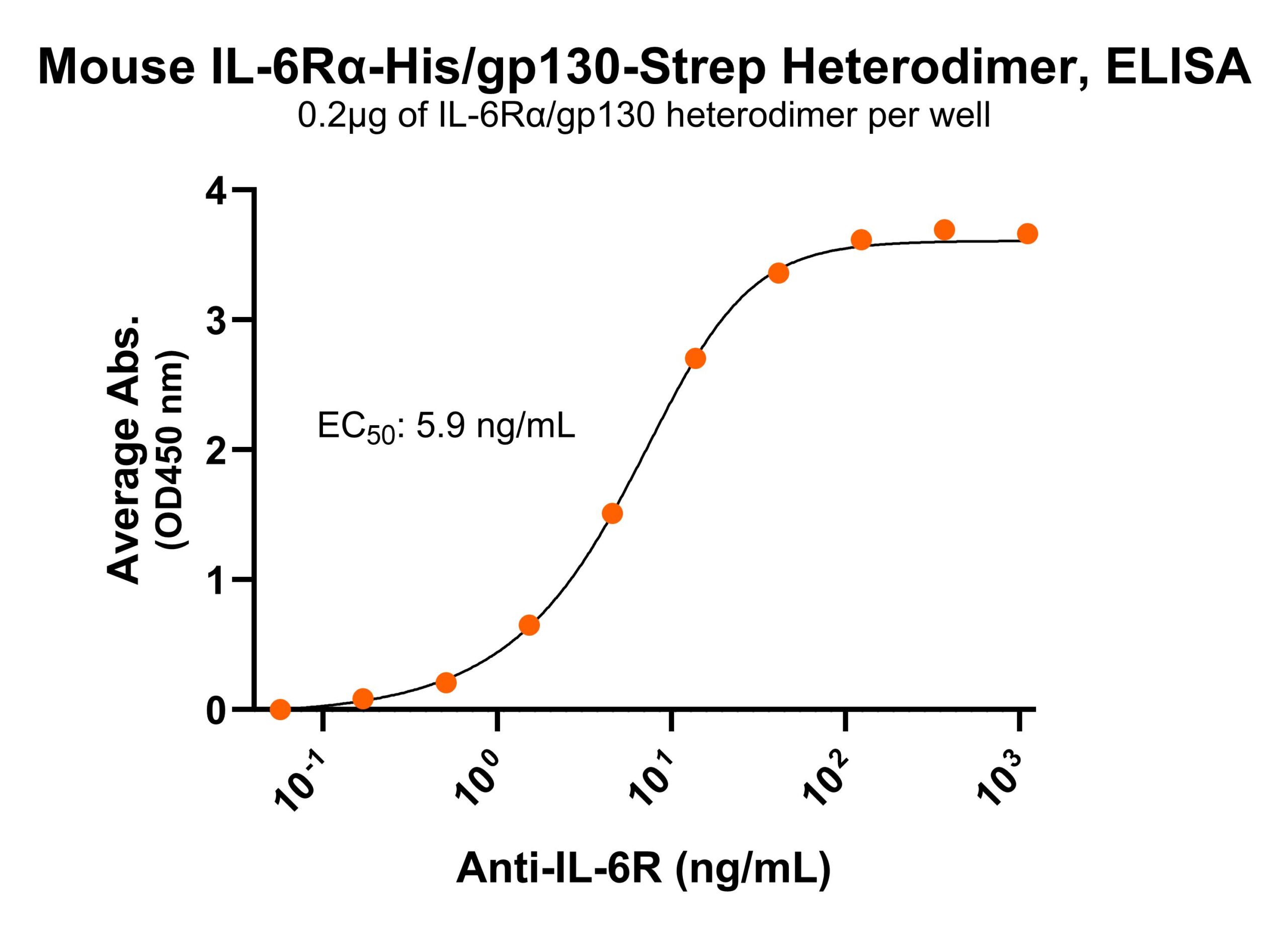 Immobilized mouse IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25256-A1B6) at 2 μg/mL (100 μL/well) can bind anti-mouse IL-6R polyclonal antibody with half maximal effective concentration (EC50) range of 2.9-11.8 ng/mL (QC tested).
Immobilized mouse IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25256-A1B6) at 2 μg/mL (100 μL/well) can bind anti-mouse IL-6R polyclonal antibody with half maximal effective concentration (EC50) range of 2.9-11.8 ng/mL (QC tested).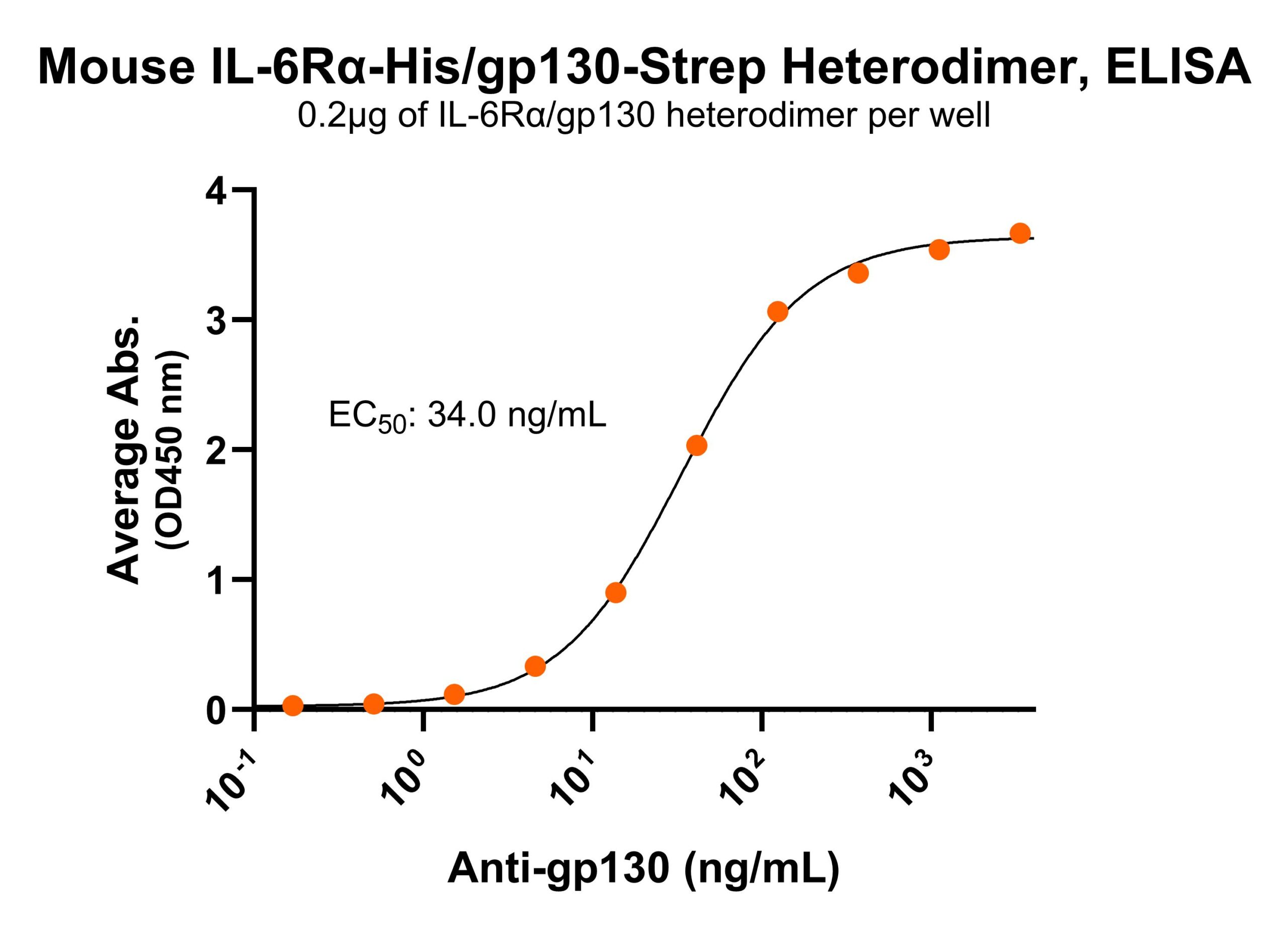 Immobilized mouse IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25256-A1B6) at 2 μg/mL (100 μL/well) can bind anti-mouse gp130 polyclonal antibody with half maximal effective concentration (EC50) range of 17-68 ng/mL (QC tested).
Immobilized mouse IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25256-A1B6) at 2 μg/mL (100 μL/well) can bind anti-mouse gp130 polyclonal antibody with half maximal effective concentration (EC50) range of 17-68 ng/mL (QC tested).