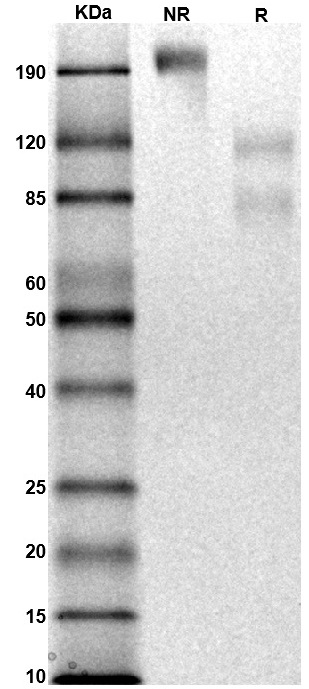
 MW: Molecular Weight marker reduced condition
NR: IL-6Rα/gp130 heterodimer under non-reduced condition
R: IL-6Rα/gp130 heterodimer under reduced condition
MW: Molecular Weight marker reduced condition
NR: IL-6Rα/gp130 heterodimer under non-reduced condition
R: IL-6Rα/gp130 heterodimer under reduced condition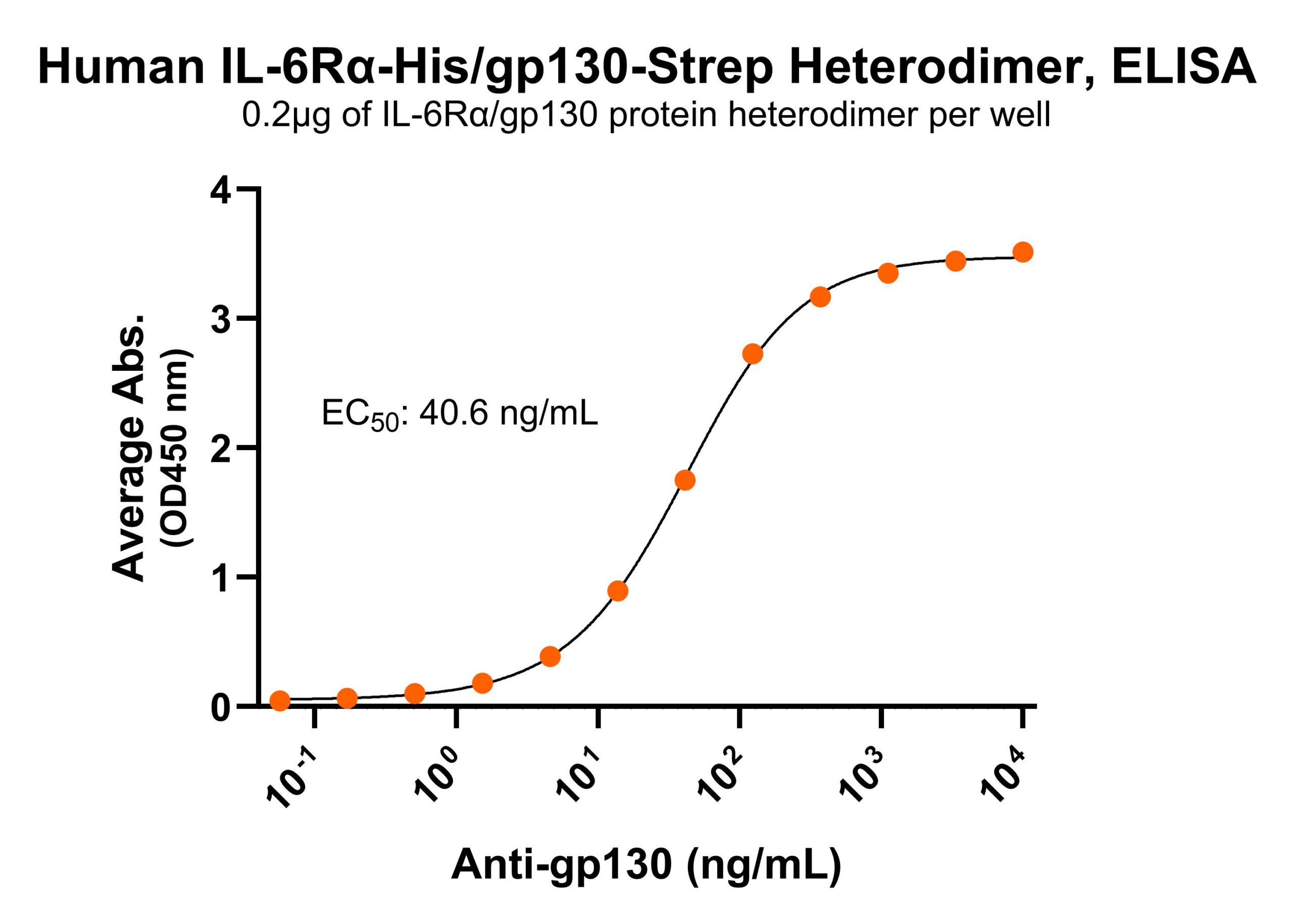 Immobilized human IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25249-A1B6) at 2 μg/mL (100 μL/well) can bind anti-human gp130 monoclonal antibody with half maximal effective concentration (EC50) range of 20.3-81.2 ng/mL (QC tested).
Immobilized human IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25249-A1B6) at 2 μg/mL (100 μL/well) can bind anti-human gp130 monoclonal antibody with half maximal effective concentration (EC50) range of 20.3-81.2 ng/mL (QC tested).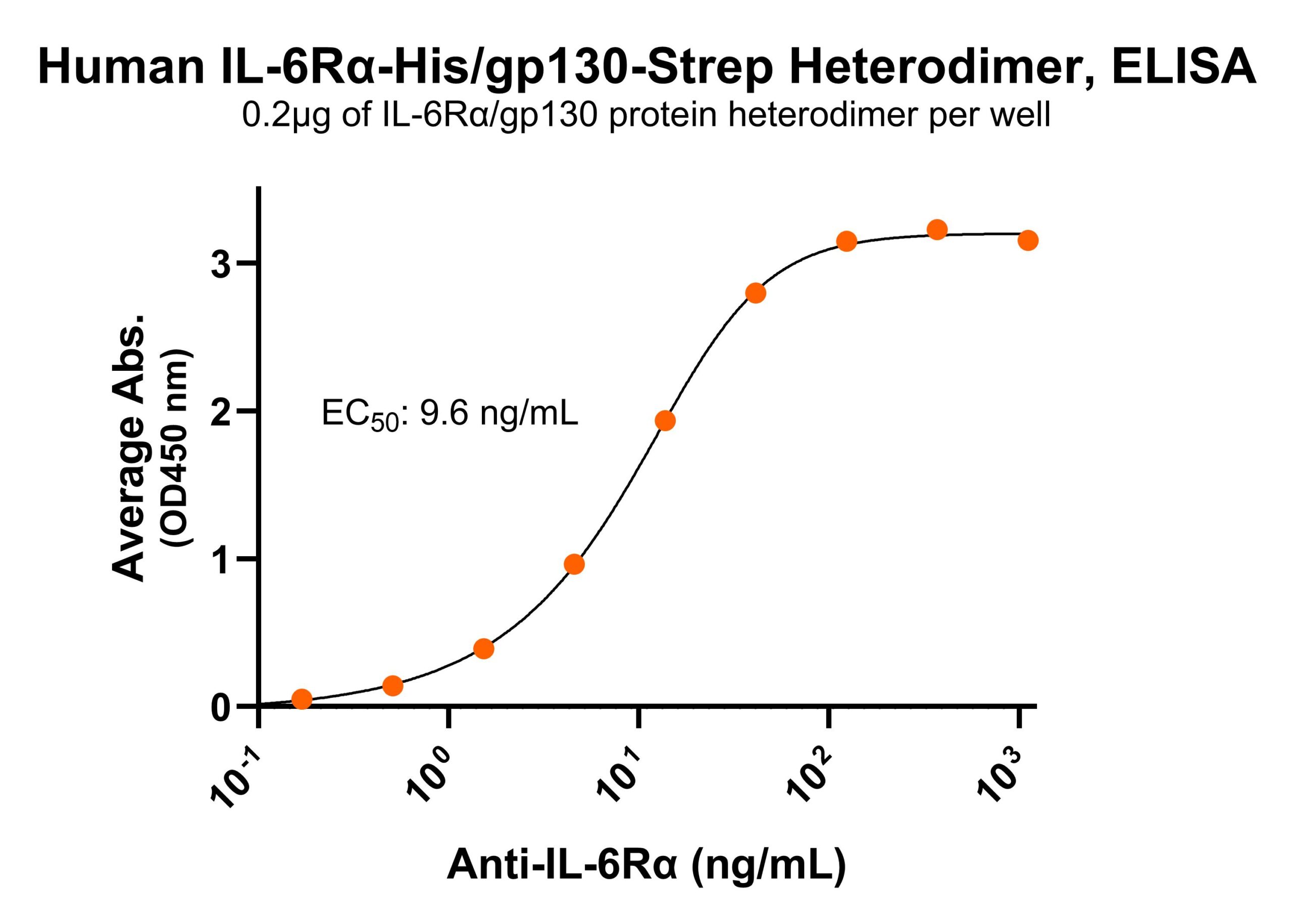 Immobilized human IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25249-A1B6) at 2 μg/mL (100 μL/well) can bind anti-human IL-6Rα monoclonal antibody with half maximal effective concentration (EC50) range of 4.8-19.2 ng/mL (QC tested).
Immobilized human IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25249-A1B6) at 2 μg/mL (100 μL/well) can bind anti-human IL-6Rα monoclonal antibody with half maximal effective concentration (EC50) range of 4.8-19.2 ng/mL (QC tested).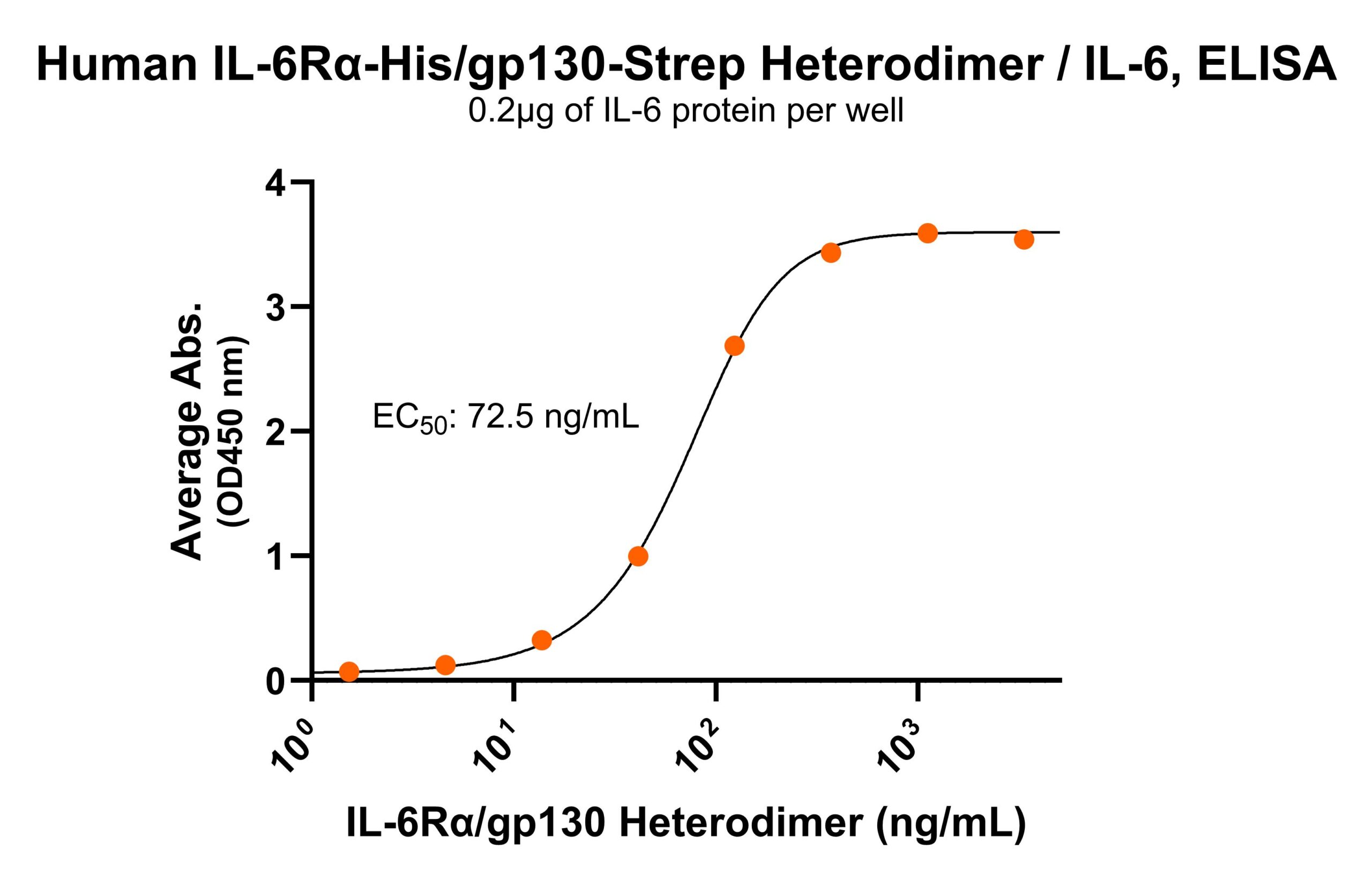 Immobilized human IL-6 at 2 μg/mL (100 μL/well) can bind human IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25249-A1B6) with half maximal effective concentration (EC50) range of 36.3-145.1 ng/mL (QC tested).
Immobilized human IL-6 at 2 μg/mL (100 μL/well) can bind human IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25249-A1B6) with half maximal effective concentration (EC50) range of 36.3-145.1 ng/mL (QC tested).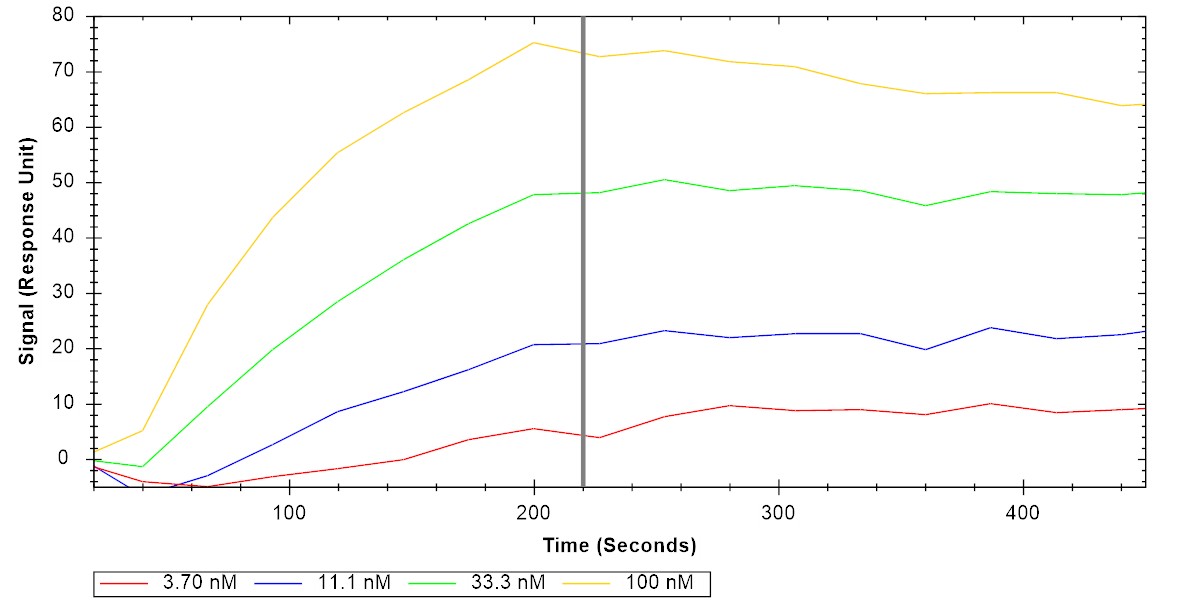 Immobilized human IL-6 can bind human IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25249-A1B6) with a KD of 0.7-2.6 nM as determined by LSPR (Nicoya Alto).
Immobilized human IL-6 can bind human IL-6Rα/gp130 protein heterodimer, His and Strep-tag (CSP-25249-A1B6) with a KD of 0.7-2.6 nM as determined by LSPR (Nicoya Alto).For Research Use Only (RUO)