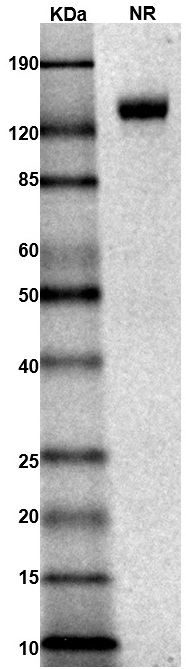
 MW: Molecular Weight marker reduced condition
NR: IL-4Rα dimer under non-reduced condition
MW: Molecular Weight marker reduced condition
NR: IL-4Rα dimer under non-reduced condition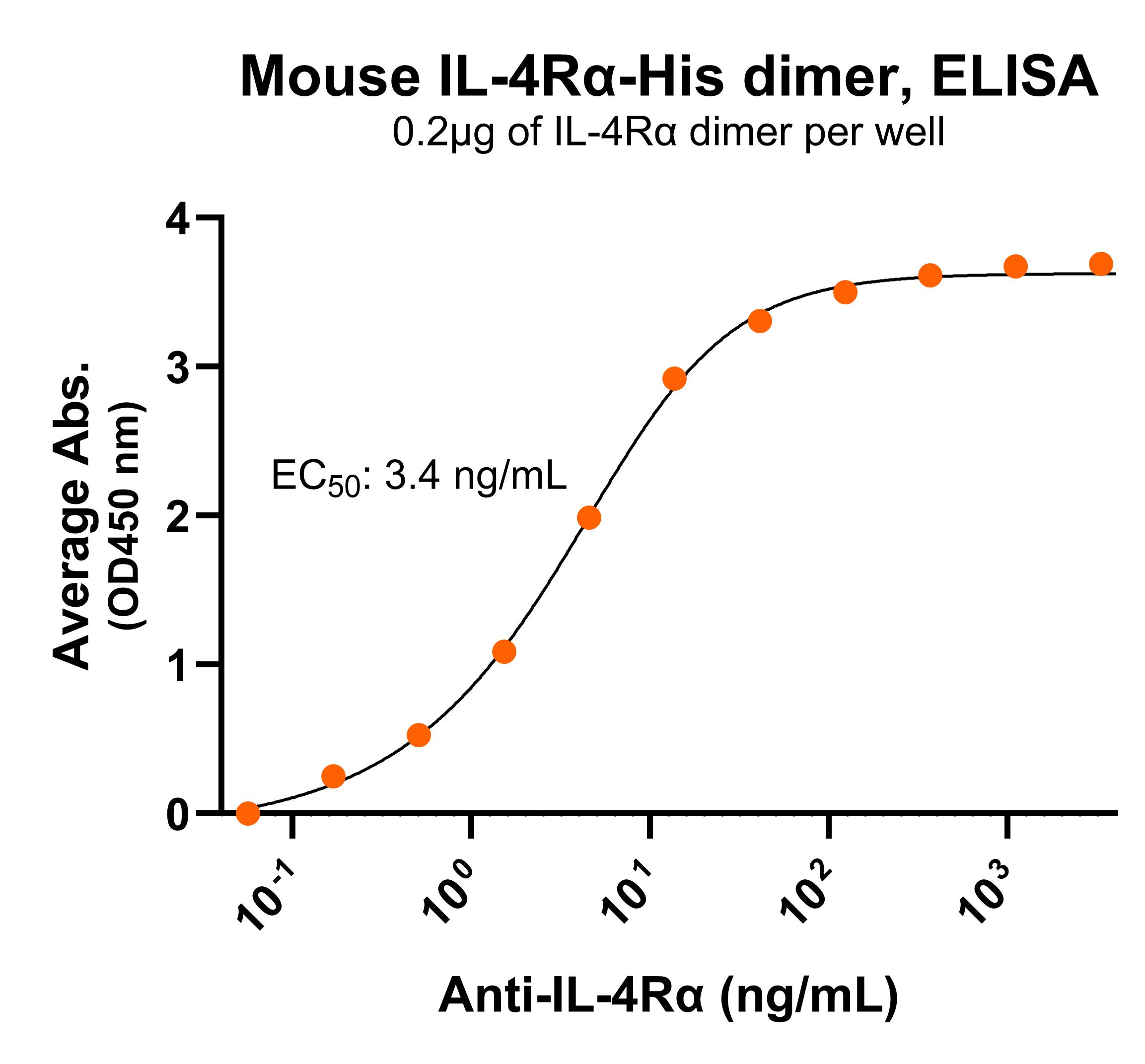 Immobilized mouse IL-4Rα protein dimer, His tag (Cat. No. CSP-25220-01) can bind anti-mouse IL-4Rα polyclonal antibody with half maximal effective concentration (EC50) range of 1.7-6.9 ng/mL (QC tested).
Immobilized mouse IL-4Rα protein dimer, His tag (Cat. No. CSP-25220-01) can bind anti-mouse IL-4Rα polyclonal antibody with half maximal effective concentration (EC50) range of 1.7-6.9 ng/mL (QC tested).For Research Use Only (RUO)